Background
In pediatric nutrition research, the artificially reared (AR) pig has become a vital model for evaluating the safety and efficacy of bioactive components in infant formula. However, the absence of standardized growth references has made it challenging to assess whether dietary interventions impact growth within normal biological ranges.
What we did
This study addressed the gap by compiling data from 22 studies, spanning a decade, to develop a reference database for typical body and organ growth in artificially reared pigs. The resulting growth charts and organ weight distributions now serve as essential benchmarks for researchers evaluating new dietary interventions in preclinical studies.
You can read the full article published in Frontiers in Pediatrics here.
Disclaimer: Reckitt provided funding to Traverse Science for conceptualization, execution, and administration of the project. Aside from project funding and employment of some of the authors, Reckitt had no other involvement in this publication.
Key Takeaways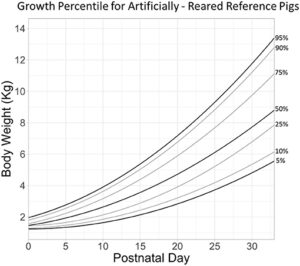
- Body Weight Growth Patterns
- Data from 786 pigs generated growth percentile charts analogous to human infant growth curves.
- Growth curves enabled the identification of cohorts of pigs that grew abnormally slow or fast.
- Organ Development Benchmarks
- A weight-for-age chart was created for body and organ weights, including the brain, liver, and intestine.
- Normalized organ weights (% of total body weight) showed minimal variation based on rearing environment or sex, further supporting the use of a unified reference dataset.
- Advanced Analytical Techniques
- Traditional univariate assessments of organ weights often miss subtle but significant biological changes.
- This study applied bivariate regression analyses, revealing critical insights, such as:
- Choline-deficient pigs exhibited higher relative brain weights due to reduced body mass, not brain overgrowth.
- Phthalate-exposed pigs displayed liver development abnormalities undetected by conventional analysis.
- Impact on Biomedical Research
- The reference database enables researchers to identify outliers in pig growth and organ development, aiding in safety assessments of infant nutrition products.
- By standardizing growth expectations, this resource enhances the interpretation of preclinical trials, reducing the risk of misinterpretation due to biological variability.
Implications for Research & Industry
This database represents a major step toward standardizing the AR pig as a biomedical model. It offers a dynamic tool for:
- Evaluating novel dietary ingredients in infant formula.
- Benchmarking organ growth and development for safety assessments.
- Improving the predictive power of pig models for human infant nutrition research.
The database is open-source and available for collaboration, encouraging broader data sharing to refine and expand these benchmarks.
By establishing a comprehensive growth reference for AR pigs, this study enhances the utility of the pig model in pediatric nutrition research. It ensures that future studies can more accurately interpret biological significance in growth and organ development—critical for the substantiation of safe and effective infant nutrition products.
Looking for expert insights into preclinical research and nutrition safety assessments? Contact us today to learn how our data-driven solutions can elevate your research.

About the Authors:
Original article written by Vinh H. Vu, Sharon M. Donovan, Laruen R. Brink, Qian Li, Gabriele Gross, Ryan N. Dilger, and Stephen A. Fleming. See full author details in the article.
Vu VH, Donovan SM, Brink LR, et al. (2021) Developing a Reference Database for Typical Body and Organ Growth of the Artificially Reared Pig as a Biomedical Research Model. Front. Pediatr. 9, 746471.
