Is the secret to a high IQ hiding in your gut? Hyperbole aside, modulation of the microbiome via prebiotics and/or probiotics have been shown to alter behavior, increase learning and memory, or lessen anxiety. The discovery of these effects has prompted an interest in the bidirectional communication between the gut and the brain, or the gut-brain-axis. How is it that prebiotics, which feed specific bacteria in the gut, affect such a distant organ like the brain? Some of the best evidence has been generated for the human milk oligosaccharide 2'fucosyllactose.
To stay updated with our blog, subscribe below!
A primer on synaptic plasticity
Synaptic plasticity is the ability of the brain to be dynamic, where synaptic connections may be made stronger or weaker to promote memory formation, which are key components for learning and memory. When studying synaptic plasticity, scientists frequently assess long-term potentiation (LTP). Memories are thought to be encoded in neurons by increases in the frequency they fire at (synaptic strength). In other words, when a neuron “holds a memory” after it “learned it”, it fires stronger and faster when it “receives information about that memory”. This synaptic plasticity, in other words, is a change from normal firing to a persistent stronger firing between neurons and is known as LTP2. This effect can be induced in a lab setting and is most commonly demonstrated in the hippocampus, an area of the brain critical for consolidating short-term memories into long-term memories.
Neuroscientists commonly use methods like the ones described in Vasquez, et al. in order to study LTP1. They insert electrodes into the region of interest, in this case the CA1 region of the hippocampus, and with these electrodes they record neuronal activity. In order to invoke LTP in the CA1, they electrically stimulate neurons in the CA3 region of the hippocampus. Since neurons in the CA3 region synapse fire onto neurons in the CA1, they can measure how neurons in CA1 have a higher frequency of firing as a result of the electrical stimulation, and therefore invoke LTP.
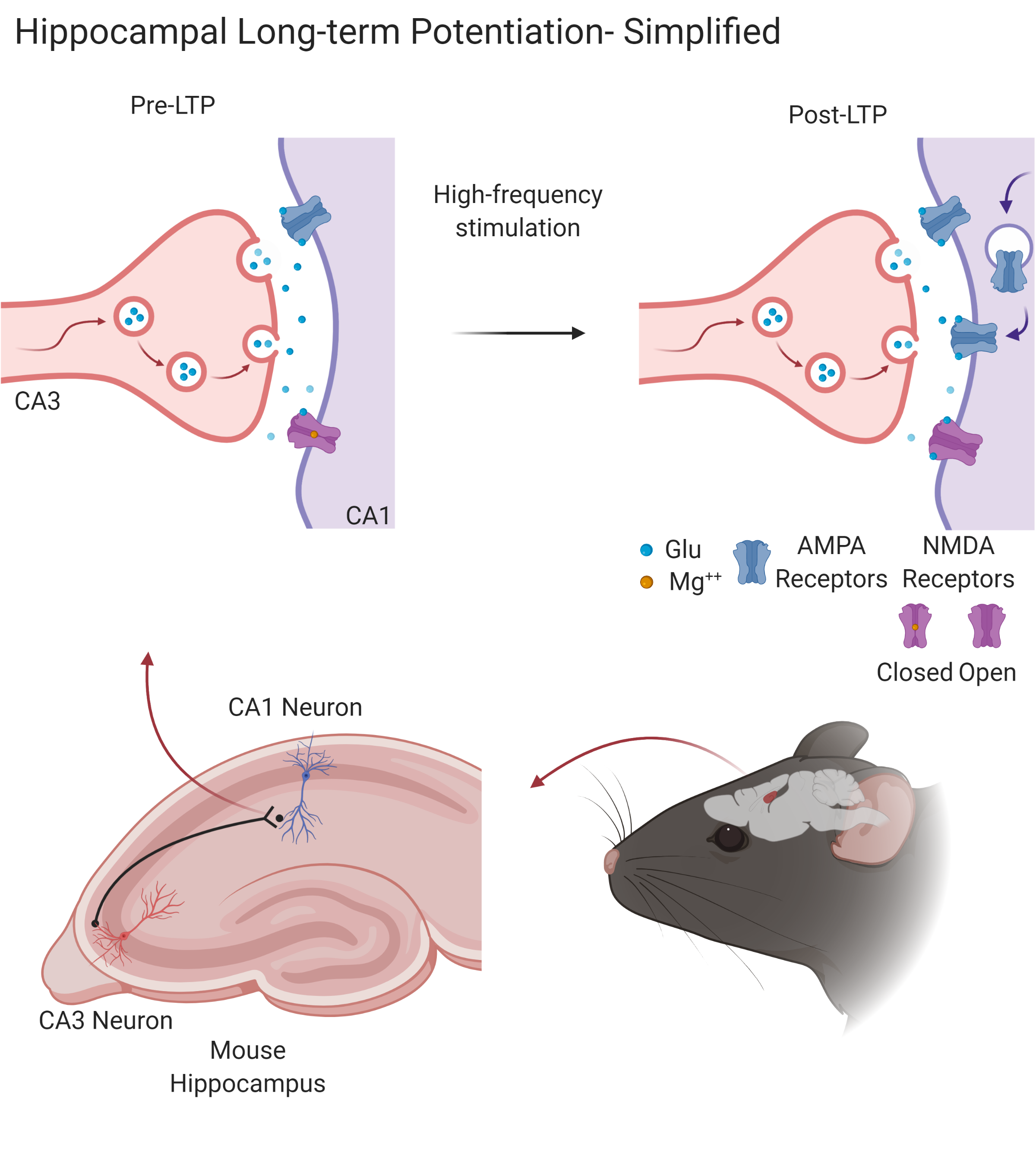
Figure 1. Simplified schematic of long-term potentiation in the hippocampus. After high-frequency stimulation of the CA1 post-synapse by the CA3 pre-synapse, more AMPA receptors are recruited to the post-synapse, the NMDA receptors open, and a molecular cascade initiates, strengthening the synapse. This is a process critical to learning and memory. Created with BioRender.com
Investigating the impact of 2′FL on the synaptic mechanisms behind memory
Molecular Changes
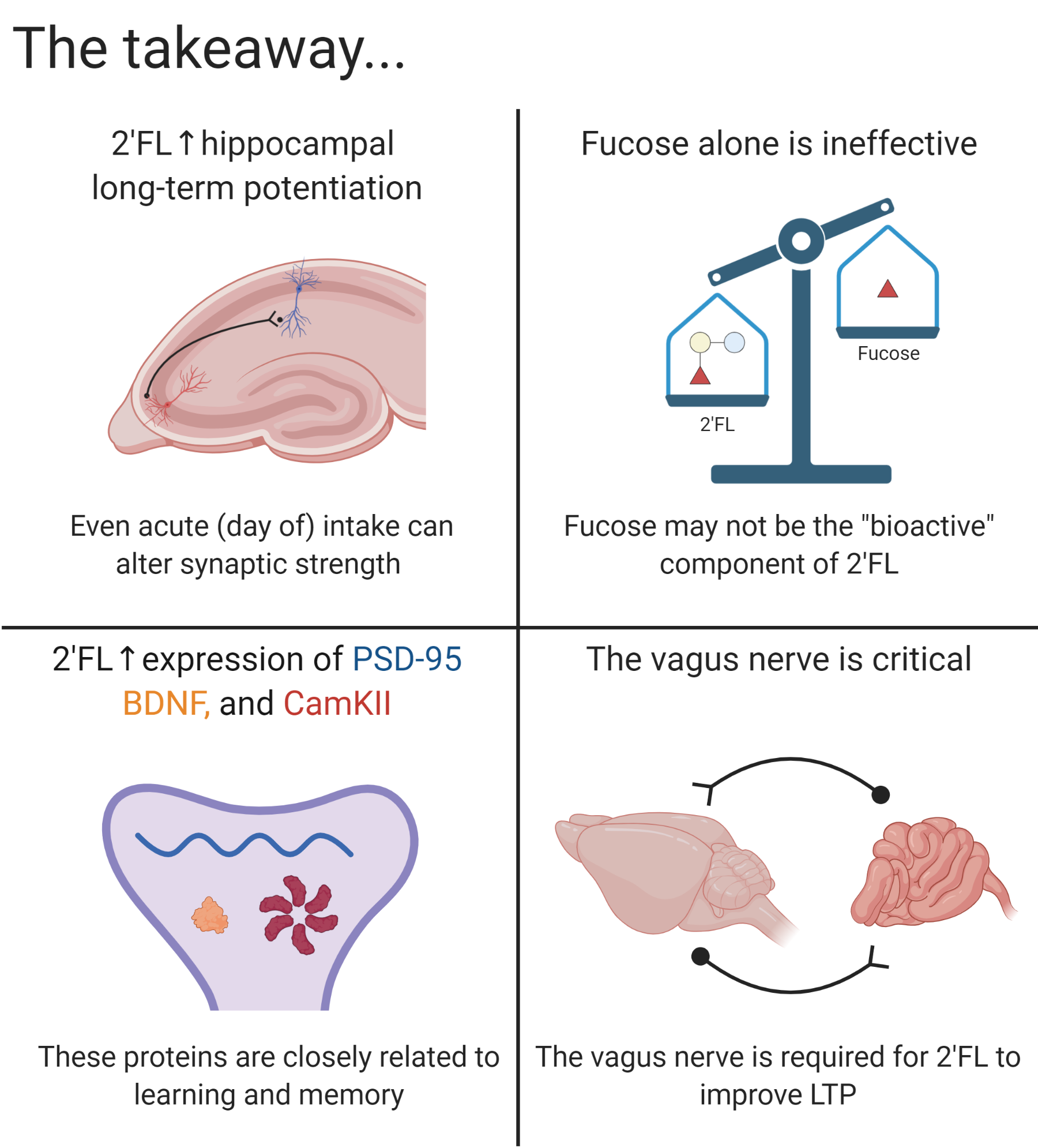
2′FL has an effect at the molecular level as well, associated with increases in key proteins involved in LTP. In order to have a look at how 2′FL enhanced LTP at a molecular level, Vázquez et al. decided to perform an experiment focusing on the postsynaptic density (PSD) of the synapse1. The PSD is an area in the synapse that is rich in proteins responsible for neuronal function3. They looked at three proteins within the PSD known for the roles in synaptic transmission: PSD-95, BDNF, and CaMKII. They specifically looked at postsynaptic densities in three key areas of the brain that are important for learning and memory: the hippocampus, the striatum, and the cerebral cortex. They found that rats supplemented with 2′FL for 5 weeks had higher levels of PSD-95, CaMKII, and BDNF in the hippocampus; higher levels of PSD-95 in the frontal cortex; and higher levels of BDNF in the striatum.
Immunostaining of the hippocampus, the striatum, and the cerebral cortex revealed that in the brains of the 2′FL fed rats, PSD-95 was not only present in the cell body, but also in the neurites. Control rats did have PSD-95 expression in the cell body as well, although significantly less compared to experimental rats, and they did not have PSD-95 expression in the neurites. Because PSD-95, CaMKII, and BDNF are markers of synapses and neuronal function, and not markers for proliferation of new neurons or synapses, we cannot conclude if new neurons or synapses were formed4. However, if we assume a similar number of synapses between the 2′FL group and control (which may be an incorrect assumption), and because we see stronger expression of proteins in the 2′FL groups as well as localization of protein in the neurites- not just specific to the cell body, these studies suggest that there are more proteins in the existing synapses.
As mentioned earlier, PSD-95, CaMKII, and BDNF are essential proteins associated with LTP. These findings together with the results from the previous experiments (where mice fed 2′FL for 12 weeks had stronger LTP that lasted for up to 3 days after induction) suggest that changes in protein expression are transient and acute, upregulated when receiving the appropriate conditions (2′FL and proper electrical stimulation) and downregulated when these conditions are no longer present (hence the lack of LTP after 3 days of LTP induction in the 12 week 2′FL mice).
2′FL as a whole is key for synaptic changes
The vagus nerve could be the link between prebiotics and the brain
One possible explanation is the vagus nerve. The vagus nerve is the longest cranial nerve present in the body, connecting from the brain stem all the way down to the gut. The vagus nerve sends and receives information from the brain to the gut and vice versa9. Additionally, vagus nerve stimulation in rats elicits LTP in the hippocampus10. Vázquez et al. explored if the vagus nerve is required for 2’FL to improve LTP7. As we mentioned before, they induced LTP in rats and those rats that were fed a diet containing 2’FL demonstrated larger LTP compared to rats that were fed standard rat chow or rat chow containing fucose. However, they showed that these positive effects on LTP were lost when severing the vagus nerve (also referred to as a vagotomy). Not only did the positive effect of 2’FL on LTP disappear, but also every group that received a vagotomy demonstrated significantly less evoked LTP compared to their control group. They also tested their hypothesis using a behavioral experiment called the fixed ratio 1 (1 lever press = 1 food pellet reward) lever pressing task. Rats that were fed 2’FL and had an intact vagus nerve learned the task quicker and pressed the lever more times compared to the rest of the groups: 2’FL + vagotomy, control + vagotomy, and control + sham.
These findings demonstrate that both an intact vagus nerve as well as consumption of 2′FL need to be present in order to elicit LTP and enhance performance in rodents. In other words, it’s not about a direct effect on the brain (absorption of 2′FL, components of 2′FL like fucose, or metabolites from bacterial fermentation), it is an indirect effect through the vagus nerve. At the very least, the vagus nerve is required to be intact to see an effect, though we are unsure if it is the only relevant mechanism. In other words, the vagus nerve is required, but we don’t know if it’s a sufficient mechanism to describe the phenomenon.
Limitations
Take-home message
Vázquez et al. used LTP (an important process for memory formation) and behavioral measures to demonstrate that 2’FL facilitates learning and memory in rodents1,7. Furthermore, they demonstrated that it is the 2’FL molecule as a whole that mediates this effect, and not just fucose. They demonstrated that rodents fed 2’FL had higher concentrations of BDNF, PSD-95, and CamKII in the hippocampus, revealing molecular changes important in learning and memory. Furthermore, they showed that the vagus nerve plays a key role in order for 2’FL to exert a beneficial effect on learning and memory. Without the vagus nerve, the positive effect 2’FL has on learning and memory would not be possible.


About the authors:
Jessica P. Caballero Feliciano is a Puerto Rican neuroscientist that believes science should be accessible to all. She did her B.A. in Psychology at the University of Puerto Rico- Mayagüez, where she worked on a project that developed and standardized the first cognitive assessment test and intervention for Spanish-speaking children. She is currently finishing her Ph.D. in Neuroscience and Behavior at the University of Massachusetts Amherst where she studies neuronal circuits involved in motivation and reward seeking behaviors in rodents. Connect with her on LinkedIn.
Stephen Fleming is President and Co-founder of Traverse Science. He believes science doesn’t have to be hard and should accelerate business, not slow it down. He has a background in neuroscience and nutrition from the University of Illinois at Urbana-Champaign where he studied oligosaccharide intake and brain development for his PhD. If you want to learn more, follow him and Traverse Science on LinkedIn, and connect with us at engage@traversescience.com
About the company: Traverse Science is a nutrition consulting firm working with ingredient suppliers and consumer packages goods companies in the human and animal nutrition space. We work with clients to get science done, whether that means organizing and conducting a study, analyzing new or long-forgotten data, or writing a manuscript for peer review or guidance document for internal use. As teams change, time runs short, or projects pivot, we provide the muscle and the know-how to finish your nutrition science and get your projects out the door – whatever that means for you. We believe that science doesn’t have to be hard, and we’re here to make it easy.
References:
- Vázquez, E. et al. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. The Journal of Nutritional Biochemistry 26, 455–465 (2015). doi: 10.1016/j.jnutbio.2014.11.016
- Purves, D. et al. Long-Term Synaptic Potentiation. Neuroscience. 2nd edition (2001). ISBN-10: 0-87893-742-0
- Dosemeci, A., Weinberg, R. J., Reese, T. S. & Tao-Cheng, J.-H. The Postsynaptic Density: There Is More than Meets the Eye. Front. Synaptic Neurosci. 8, (2016). doi: 10.3389/fnsyn.2016.00023
- Kuhn, H. G., Eisch, A. J., Spalding, K. & Peterson, D. A. Detection and Phenotypic Characterization of Adult Neurogenesis. Cold Spring Harb Perspect Biol 8, (2016). doi: 10.1101/cshperspect.a025981
- Kobata, A. & Ginsburg, V. Oligosaccharides of Human Milk III. Isolation and Characterization of a New Hexasaccharide, Lacto-N-Hexaose. J. Biol. Chem. 247, 1525–1529 (1972). ISSN: 0021-9258, 1083-351X
- Jork, R., Grecksch, G. & Matthies, H. Impairment of glycoprotein fucosylation in rat hippocampus and the consequences on memory formation. Pharmacology Biochemistry and Behavior 25, 1137–1144 (1986). doi: 10.1016/0091-3057(86)90100-0
- Vázquez, E. et al. Dietary 2’-Fucosyllactose Enhances Operant Conditioning and Long-Term Potentiation via Gut-Brain Communication through the Vagus Nerve in Rodents. PLoS ONE 11, e0166070 (2016). doi: 10.1371/journal.pone.0166070
- Gibson, G. R. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology 14, 491–502 (2017). doi: 10.1038/nrgastro.2017.75
- Fülling, C., Dinan, T. G. & Cryan, J. F. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron 101, 998–1002 (2019). doi: 10.1016/j.neuron.2019.02.008
- Zuo, Y., Smith, D. C. & Jensen, R. A. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiology & Behavior 90, 583–589 (2007). doi: 10.1016/j.physbeh.2006.11.009
- Berger, P. K. et al. Human milk oligosaccharide 2’-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS One 15, e0228323 (2020). doi: 10.1371/journal.pone.0228323
- Jorgensen, J. M. et al. Associations of human milk oligosaccharides and bioactive proteins with infant growth and development among Malawian mother-infant dyads. The American Journal of Clinical Nutrition 113, 209–220 (2021). doi: 10.1093/ajcn/nqaa272
Share this Post
